李国杰(经理):18202125732 电话:021-61062636
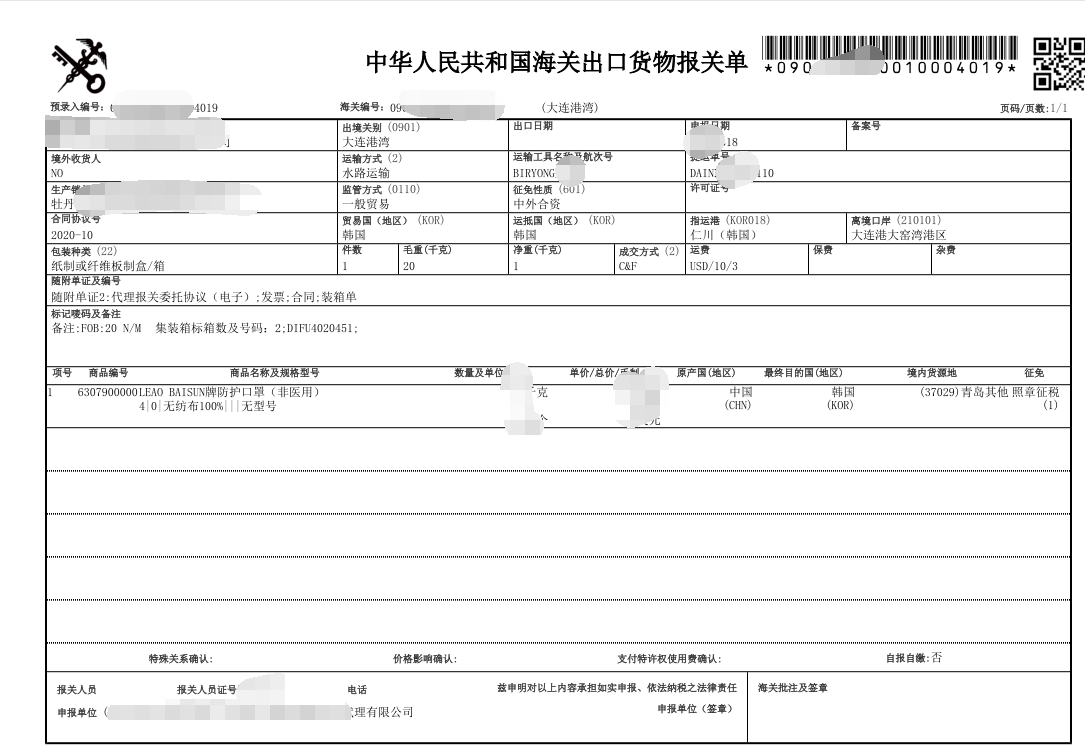
出口***物资的通关手续有什么变化?
What are the changes in the customs clearance procedures for the export of medical materials?
答:“5号公告”发布以后,企业申报出口公告范围内的5类***物资时,在通关环节与之前相比需新增提交“***器械产品注册证书”和“出口***物资声明”,其中“***器械产品注册证书”应与报关单申报信息一致。海关凭***监督管理部门批准的***器械产品注册证书验放相关出口***物资。“53号公告”发布以后, 有11类出口***物资需实施出口商品检验。此次新增实施出口商品检验的***物资不实施产地检验,申报时无需电子底账。企业需在申报时注明是否***。其中,在“5号公告”范围内的***物资,按照上述要求提交随附单据。其他出口***物资,建议企业按照《***器械分类目录》的管理类别,在向海关申报时,参照“5号公告”的要求,分别提供***器械产品注册/备案证明,以及质量安全声明。
Answer: "after announcement 5" is issued, when enterprises declare 5 types of medical materials within the scope of export announcement, they need to submit "medical device product registration certificate" and "export medical material declaration" in the customs clearance link compared with the previous one. Among them, "medical device product registration certificate" should be c***istent with the declaration information of the customs declaration. The customs shall examine and release the relevant exported medical materials with the registration certificate of medical devices approved by the drug regulatory department. After the Announcement No. 53 was issued, 11 types of exported medical materials need to be subject to export commodity inspection. The newly added medical materials subject to export commodity inspection will not be subject to origin inspection, and no electronic ledger is required for declaration. The enterprise shall indicate whether it is medical or not at the time of declaration. Among them, for medical materials within the scope of "announcement 5", the attached documents shall be submitted according to the above requirements. For other exported medical materials, it is suggested that the enterprise shall, according to the management category of the classification catalogue of medical devices, respectively provide the registration / Filing Certificate of medical device products and the quality and safety statement according to the requirements of announcement 5 when declaring to the customs.
“5号公告”和“53号公告”所列范围之外的物资出口,需要提交相关单证吗?
Do you need to submit relevant documents for the export of materials beyond the scope of "announcement 5" and "announcement 53"?
答:对于公告范围之外的非***防护用品等出口物资,无需提交***器械产品注册/备案证明和质量安全声明,通关手续按原有要求办理。
Answer: for non-medical protective articles and other export materials beyond the scope of announcement, it is not necessary to submit the registration / Filing Certificate and quality safety statement of medical device products, and the customs clearance procedures shall be handled according to the original requirements.

李国杰(经理):18202125732 电话:021-61062636
专注于进出口的物流师
在进出口清关的过程中遇到任何问题,都可咨询我,免费为您提供***解答。15年***进口清关团队竭诚为您服务
上海|宁波|昆山|深圳|广州|厦门|成都|天津|青岛|北京|大连|***网点服务
In the process of import customs clearance, you can c***ult me and provide you with professional answers free of charge. 15 years of professional import customs clearance team dedicated to serve you
Shanghai, Ningbo, Kunshan, Shenzhen, Guangzhou, Xiamen, Chengdu, Tianjin, Qingdao, Beijing, Dalian, national network service