李国杰(经理):18202125732 电话:021-61062636
出口日本的PMDA注册***器械公司希望把产品投放到日本市场必须要满足日本的Pharmaceutical and Medical Device Act (PMD Act),在PMD Act的要求下,TOROKU注册系统要求国外的制造商必须向PMDA注册制造商信息。包装上印有ウィルスカ***ト99%的字?样?都是超过国内过滤效率95%(N95口罩)标准的***口罩!
1. ***防护口罩:符合中国GB 强制性标准,过滤效率≥95%(使用非油性颗粒物测试)。
2. N95口罩:美国NIOSH认证,非油性颗粒物过滤效率≥95%。
3. KN95口罩:符合中国GB 2626 强制性标准,非油性颗粒物过滤效率≥95%欧盟
4.必要资料(资质)
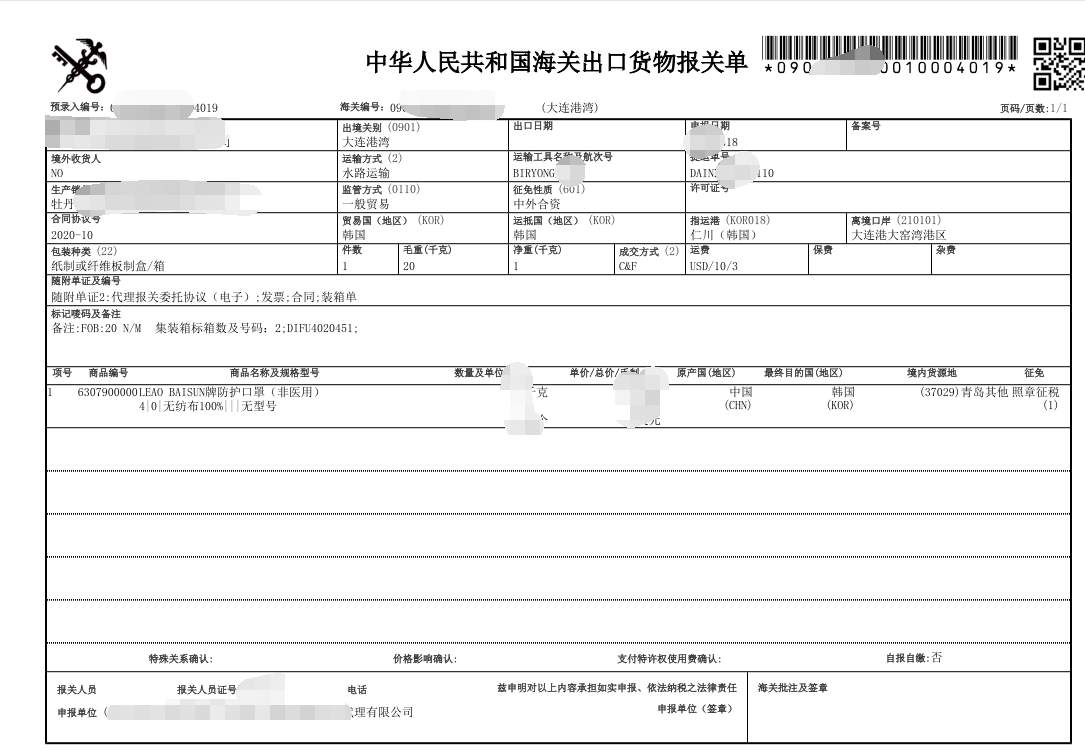
提单,箱单,***
5.在欧盟,口罩属于PPE个人防护用品,“危及健康的物质和混合物”。2019年起,欧盟新***PPE Regulation (EU) 2016/425强制执行,所有出口欧盟的口罩必须在新***的要求下获得CE认证证书。CE认证是欧盟实行的强制性产品安全认证制度,目的是为了保障欧盟***人民的生命财产安全。
1. Medical protective mask: comply with Chinese GB compulsory standard, with filtration efficiency ≥ 95% (test with non oily particles).
2. N95 respirator: NIOSH certified, the filtration efficiency of non oil particles ≥ 95%.
3. Kn95 respirator: it meets the mandatory standard of China GB 2626, and the filtration efficiency of non oil particles ≥ 95% of EU
4. Necessary information (qualification)
Bill of lading, packing list, invoice
5. In the European Union, masks are PPE personal protective equipment, "substances and mixtures endangering health". From 2019, the new EU regulation PPE regulation (EU) 2016 / 425 is enforced. All masks exported to the EU must obtain CE certification under the requirements of the new regulation. CE certification is a compulsory product safety certification system implemented by EU, which aims to protect the life and property safety of EU people.

美国目前并没有***禁止口罩出口的消息。建议中国卖家及时关注国内外防疫物资出口资质认证和监管要求,避免造成产品被扣押和退回的风险。属于***器械类的口罩,不同***的资质和要求都不相同,这点卖家们一定要注意。
李国杰(经理):18202125732 电话:021-61062636
专注于进出口的物流师
在进出口清关的过程中遇到任何问题,都可咨询我,免费为您提供***解答。15年***进口清关团队竭诚为您服务
上海|宁波|昆山|深圳|广州|厦门|成都|天津|青岛|北京|大连|***网点服务
In the process of import customs clearance, you can c***ult me and provide you with professional answers free of charge. 15 years of professional import customs clearance team dedicated to serve you
Shanghai, Ningbo, Kunshan, Shenzhen, Guangzhou, Xiamen, Chengdu, Tianjin, Qingdao, Beijing, Dalian, national network service