李国杰(经理):18202125732 直线:021-61062636
海关监管
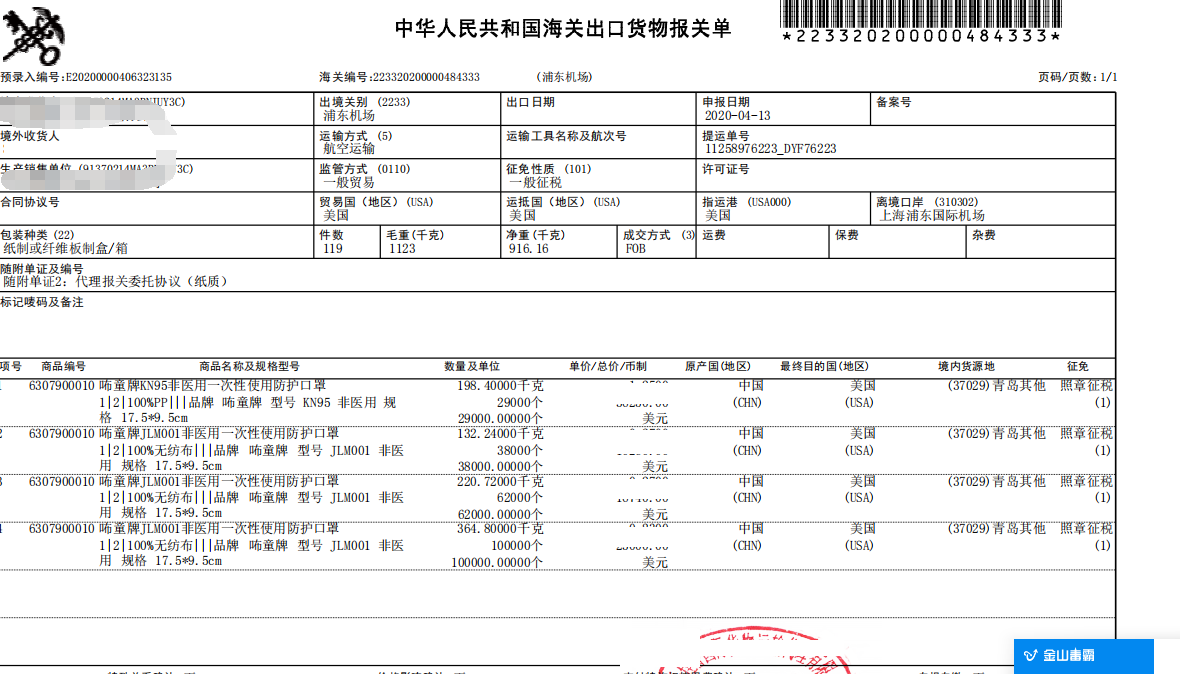
为进一步加强新冠***疫情期间出口防疫物资质量安全监管,避免出口不合格防疫物资,海关近期在货物贸易渠道,开展了出口口罩、防护服、护目镜、手套、呼吸机等防疫物资质量安全监管专项工作。
对存在质量安全问题的出口防疫物资依法依规实施快速处置,对发现企业有出口防疫物资伪瞒报、夹藏夹带、掺杂掺假、以假充真、以次充好或者以不合格冒充合格等***行为的,将严格依法依规进行处理。
欧盟***器械***MDR(EU 2017/745)于2017年5月25日正式生效,与旧的***器械指令MDD(93/42/EEC)交替使用过渡期为三年。因此,按照旧的***器械指令MDD(93/42/EEC)所获CE认证的口罩等***器械产品,需面临换版问题。对已经在欧盟渠道正式上市的产品,MDD(93/42/EEC)指令CE证书可以保持到2024年5月26日,今年5月26日前未能上市的产品,原则上应将旧的MDD证书重新申请调整到MDR版本。MDR的审核流程和要求更为复杂繁琐,认证周期更长,企业应引起注意。
Customs supervision
For novel coron***irus pneumonia epidemic prevention and quarantine materials to improve the quality and safety supervision during the epidemic situation, and to ***oid the export of unqualified anti epidemic materials, the Customs recently carried out export safety management of special materials for export of respirators, protective clothing, goggles, gloves and ventilator.
The export epidemic prevention materials with quality and safety problems shall be disposed quickly in accordance with the laws and regulati***, and the enterprises found to h***e the illegal beh***iors such as false report of export epidemic prevention materials, entrapment and entrainment, ***eration, fake as true, inferior as good or unqualified as qualified shall be dealt with in strict accordance with the laws and regulati***.
The EU medical device regulation MDR (EU 2017 / 745) came into force on May 25, 2017, and the transition period between MDR and the old medical device directive MDD (93 / 42 / EEC) is three years. Therefore, according to the old medical device directive MDD (93/42/EEC) obtained CE certification of medical device products such as masks, we need to face the issue of version change. For the products that h***e been officially launched in the EU channel, the CE certificate of MDD (93 / 42 / EEC) directive can be maintained until May 26, 2024. For the products that h***e not been launched before May 26, 2024, in principle, the old MDD certificate should be reapplied and adjusted to the MDR version. The audit process and requirements of MDR are more complex and tedious, and the certification cycle is longer, so enterprises should pay attention to it.

李国杰(经理):18202125732 直线:021-61062636
专注于进出口的物流师
在进出口清关的过程中遇到任何问题,都可咨询我,免费为您提供***解答。15年***进口清关团队竭诚为您服务
上海|宁波|昆山|深圳|广州|厦门|成都|天津|青岛|北京|大连|***网点服务
In the process of import customs clearance, you can c***ult me and provide you with professional answers free of charge. 15 years of professional import customs clearance team dedicated to serve you
Shanghai, Ningbo, Kunshan, Shenzhen, Guangzhou, Xiamen, Chengdu, Tianjin, Qingdao, Beijing, Dalian, national network service